Natural Fertility Boosters for Luteal Phase Defect

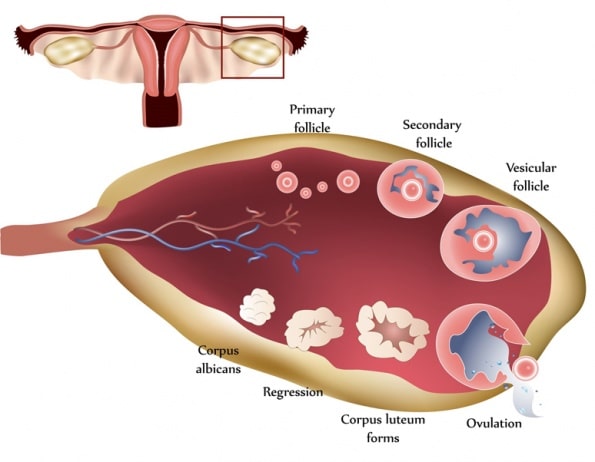
The luteal phase is the stage of a woman’s cycle beginning at the time of ovulation and ending when the menses begin. During this time, the follicle undergoes its dramatic transformation into the corpus luteum, which will secrete predominantly progesterone. The distinct shift in the hormonal climate during the luteal phase alters the structure of the endometrium, allowing the embryo to successfully implant. Without a healthy luteal phase implantation can’t happen, and so effective luteal phase defect treatment is crucial to restore healthy fertility.
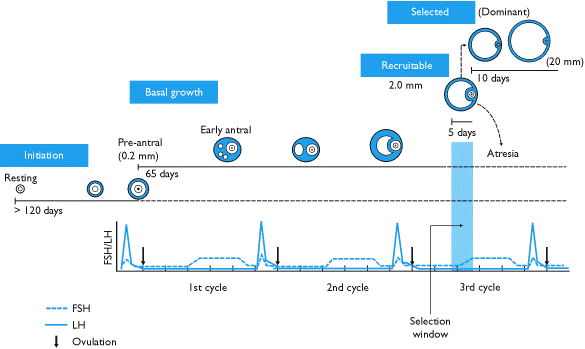
There is a misconception that a follicle develops in a single menstrual cycle. Although the luteal phase typically lasts 2 weeks, ovulation and the formation of the corpus luteum are the culmination of a much longer period of preparation. The process of folliculogenesis begins around 375 days before ovulation with the recruitment of primordial follicles that have been dormant since birth.
At birth, the primordial follicles contain the oocyte surrounded by granulosa cells. Once recruited, it takes more than 300 days for a follicle to complete the preantral period, during which time granulosa cells replicate and theca cells are recruited. When these follicles finally reach the beginning of the 28-day cycle that they have been preparing for, they will compete for follicle-stimulating hormone to become the dominant follicle. During the first 2-week period of the cycle, theca cells produce androgens, and granulosa cells aromatize androgens to estrogen.
During the follicular phase, estrogen stimulates proliferation of endometrial cells. In the luteal phase, progesterone differentiates the endometrial stroma, increases glandular secretions, and changes the pattern of uterine proteins to produce an environment supportive of early embryonic development. Progesterone is also anti-inflammatory and induces relaxation of the myometrium.
Formation of the Corpus Luteum and the Luteal Phase
By the end of the follicular phase, the luteinizing hormone surge results in ejection of the oocyte and transforms the remaining follicle into the corpus luteum; small and large luteal cells are formed from theca and granulosa cells, respectively. The small luteal cells secrete small amounts of progesterone and convert cholesterol to androgens. The large luteal cells produce 6 to 8 times the progesterone of the theca cells and aromatize androgens to estrogen.
Factors Influencing Luteal Cell Function
Membranes of luteal cells are in proximity to capillaries. This provides for the high metabolism of the corpus luteum, which consumes 2 to 6 times more oxygen per unit weight than the liver, kidney, or even the heart. As you can imagine, the promotion of circulation is essential for healthy luteal cell hormone production.
Production of progesterone depends on mitochondrial function in the luteal cells and a host of hormonal factors. It also depends on availability of its precursors cholesterol and pregnenolone. Hormones that specifically support the growth or function of the corpus luteum include luteinizing hormone, growth hormone (GH), insulin-like growth factor 1, prostaglandin E2, and prostacyclin.1
Thyroid and Adrenal Function and Relationships to Progesterone
Thyroid function is essential for development and growth of the follicle. Thyroid hormones work synergistically with follicle-stimulating hormone to develop healthy granulosa cells. Thyroid hormones have also been found to augment steroidogenesis from granulosa and large luteal cells.2
The adrenal glands produce a small amount of progesterone, which they use mainly in the synthesis of cortisol. In situations of stress where high levels of cortisol are produced by the adrenals, more progesterone is also needed as a substrate but this does not directly affect levels after ovulation since this occurs in the adrenals alone.
However, stress when significant can block ovulation altogether by downregulating ovulation through the hypothalamus in the brain. This condition is known as hypothalamic amenorrhea and is simple to differentiate as a cause when looking at lab testing.
Diagnostics, Signs, and Symptoms
There are several diagnostic markers that we can use to detect luteal phase deficiencies. These include the following:
- Basal body temperature charting can pinpoint a luteal phase defect (<shorter than 12 days), which indicates that the corpus luteum has degenerated prematurely.
- Basal body temperature charting can detect a premature drop in or instability of luteal phase temperature. Luteal phase temperature should be high and steady. If not, this indicates poor quality or premature degeneration of the corpus luteum.
- Bleeding or spotting in the luteal phase or before the onset of the period also indicates poor quality or premature degeneration of the corpus luteum.
- Low serum progesterone level (<20 ng/mL) or low salivary progesterone level obtained 7 days after ovulation indicates poor function of the luteal cells.
- Testing for cortisol and full thyroid panel should also be completed. Abnormalities in these profiles will aggravate luteal phase deficiencies.
Treatments – Natural Fertility Boosters
General nutrition is important. A whole-foods diet should be practiced, including plenty of high-quality protein and avoidance of hormone disruptors. Foods rich in healthy fats such as avocados, nuts and seeds, free-range organic eggs, and extra-virgin coconut oil provide precursors for steroid hormones. Because folliculogenesis is a prolonged process, nutrition in the year before a cycle can affect the luteal phase.
Natural fertility boosters to be given during the entire cycle include the following:
- Extract of maritime pine bark (50 mg 3 times a day) enhances circulation, reduces clotting, and increases GH to support function of the corpus luteum.3
- Vitamin B6 has been used clinically to treat luteal phase defect. It modulates expression of receptors to hormones and progesterone.4 Vitamin B complex supports adrenal health and stress responses, so a high-quality complex should be taken daily. Add pyridoxal-5-phosphate to achieve 100 mg/d of vitamin B6.
- Melatonin (1.5-3 mg at bedtime) increases progesterone production and supports GH secretion.5-7
- Vitamin C (750 mg/d) improves progesterone levels and increases fertility in women with luteal phase defect8 and enhances cellular health.
- Increased magnesium glycinate (400 mg/d) is required during the luteal phase because of increased progesterone synthesis.9
- Omega-3 fatty acids (approximately 1200 mg of eicosapentaenoic acid and 800 mg of docosapentaenoic acid daily) are essential for follicle structure and function and for hormone production.10,11 They have been found to directly increase progesterone secretion; they enhance uterine and ovarian blood flow by increasing the ratio of prostacyclin to thromboxane.12
- Zinc picolinate (30 mg/d) increases insulin-like growth factor 1 and GH.13,14 Zinc deficiency may impair formation of the corpus luteum.
- Coenzyme Q10 (800 mg/d) or ubiquinol (300 mg/d) is recommended to support mitochondria and biosynthesis in endoplasmic reticulum for cell development and function.15
Supplements to be given in the luteal phase only of the cycle include the following:
- Vitex agnus-castus (170 mg of a 6:1 extract of Vitex fruit daily on rising) boosts progesterone production.16,17
- Bioidentical progesterone supplementation is recommended when indicated. Increased serum progesterone levels improve blood flow to the corpus luteum1 and promote changes in the endometrium that support implantation.
If indicated, the following supplements are recommended:
- Thyroid support in the form of a high-potency thyroid formula, including l-tyrosine (1000 mg/d), selenium (200 µg/d), and iodine (450 µg/d). Aim to bring thyrotropin to a level of 2.5 mIU/L or below. Avoid iodine in cases of autoimmune thyroiditis. Bioidentical hormones may be needed in some cases.
- Stress reduction and adrenal support may be required. Consider adrenal formulas with Rhodiola,Eleutherococcus, or glandulars, depending on the 4-point cortisol results.
- Thyroid support in the form of a high-potency thyroid formula, including l-tyrosine (1000 mg/d), selenium (200 µg/d), and iodine (450 µg/d). Aim to bring thyrotropin to a level of 2.5 mIU/L or below. Avoid iodine in cases of autoimmune thyroiditis. Bioidentical hormones may be needed in some cases.
- Stress reduction and adrenal support may be required. Consider adrenal formulas with Rhodiola,Eleutherococcus, or glandulars, depending on the 4-point cortisol results.
A Healthy Luteal Phase Delivers
A healthy luteal phase is an essential part of a woman’s fertility with its crucial role in the implantation of the embryo and the maintenance of early pregnancy. In the naturopathic clinic, we can promote a healthier luteal phase by cultivating high-quality luteal cells from their very beginning, enhancing the factors that influence the function of the corpus luteum, and supporting optimal hormonal balance.
References
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80(1):1-29.
- Datta M, Roy P, Banerjee J, Bhattacharya S. Thyroid hormone stimulates progesterone release from human luteal cells by generating a proteinaceous factor. J Endocrinol. 1998;158(3):319-325.
- Buz’Zard AR, Peng Q, Lau BH. Kyolic and Pycnogenol increase human growth hormone secretion in genetically-engineered keratinocytes. Growth Horm IGF Res. 2002;12(1):34-40.
- Tully DB, Allgood VE, Cidlowski JA. Modulation of steroid receptor–mediated gene expression by vitamin B6. FASEB J. 1994;8(3):343-349.
- Taketani T, Tamura H, Takasaki A, et al. Protective role of melatonin in progesterone production by human luteal cells. J Pineal Res. 2011;51(2):207-213.
- Durotoye LA, Webley GE, Rodway RG. Stimulation of the production of progesterone by the corpus luteum of the ewe by the perfusion of melatonin in vivo and by treatment of granulosa cells with melatonin in vitro. Res Vet Sci. 1997;62(2):87-91.
- Nassar E, Mulligan C, Taylor L, et al. Effects of a single dose of N-acetyl-5-methoxytryptamine (Melatonin) and resistance exercise on the growth hormone/IGF-1 axis in young males and females. J Int Soc Sports Nutr. 2007;4:14. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2174513/?tool=pubmed. Accessed June 15, 2012.
- Henmi H, Endo T, Kitajima Y, Manase K, Hata H, Kudo R. Effects of ascorbic acid supplementation on serum progesterone levels in patients with a luteal phase defect. Fertil Steril. 2003;80(2):459-461.
- Facchinetti F, Borella P, Valentini M, Fioroni L, Genazzani AR. Premenstrual increase of intracellular magnesium levels in women with ovulatory, asymptomatic menstrual cycles. Gynecol Endocrinol. 1988;2(3):249-256.
- Zachut M, Dekel I, Lehrer H, et al. Effects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte quality. J Dairy Sci. 2010;93(2):529-545.
- Coyral-Castel S, Ramé C, Fatet A, Dupont J. Effects of unsaturated fatty acids on progesterone secretion and selected protein kinases in goat granulosa cells. Domest Anim Endocrinol. 2010;38(4):272-283.
- Saldeen P, Saldeen T. Women and omega-3 fatty acids. Obstet Gynecol Surv. 2004;59(10):722-730.
- Yu ZP, Le GW, Shi YH. Effect of zinc sulphate and zinc methionine on growth, plasma growth hormone concentration, growth hormone receptor and insulin-like growth factor–I gene expression in mice. Clin Exp Pharmacol Physiol. 2005;32(4):273-278.
- Hamza RT, Hamed AI, Sallam MT. Effect of zinc supplementation on growth hormone insulin growth factor axis in short Egyptian children with zinc deficiency. Ital J Pediatr. 2012;38(1):21. http://www.ijponline.net/content/38/1/21/abstract. Accessed June 15, 2012.
- Bentinger M, Tekle M, Dallner G. Coenzyme Q: biosynthesis and functions. Biochem Biophys Res Commun. 2010;396(1):74-79.
- Ibrahim NA, Shalaby AS, Farag RS, Elbaroty GS, Nofal SM, Hassan EM. Gynecological efficacy and chemical investigation of Vitex agnus-castus L. fruits growing in Egypt. Nat Prod Res. 2008;22(6):537-546.
- Bergmann J, Luft B, Boehmann S, Runnebaum B, Gerhard I. The efficacy of the complex medication Phyto-Hypophyson L in female, hormone-related sterility: a randomized, placebo-controlled clinical double-blind study [in German]. Forsch Komplementarmed Klass Naturheilkd. 2000;7(4):190-199.